The Autogenous Vaccines Market is slated to witness an exhilaration In Upcoming Years. The current situation calls for creating value and novel services for numerous stakeholders through innovation and acquisition of capabilities for rapidly adapting to the altering circumstances. As such, the profoundness of transformation concerning organizations’ and businesses’ activities, competencies, processes, and models is expected to be seen all through in the forecast period.
Vaccination of animals is an essential part of animal health management. It helps to boost the immune system and keep animals safe from various bacterial and viral infections. Autogenous vaccines are most preferred for animal vaccination purposes. Federal guidelines state that an autogenous vaccine is made from a bacterial or viral strain of the diseased animal. Products made from these strains can only be marketed by veterinarian or farm owners from where they were isolated.
Over the years, there has been growing demand for autogenous vaccines that are derived from bacterial strains, owing to their greater efficacy as compared to viral strains. Salmonella, Staphylococcus, Haemophilus, and Mycoplasma are some of the commonly used bacterial strains to develop autogenous bacterial vaccines. Growing prevalence of bacterial infections among animals, preference toward autogenous vaccines over antibiotics, and increased funding and support for international animal disease eradication are likely to upswing demand for autogenous bacterial vaccines over the coming years.
According to a revised report published by Persistence Market Research, the autogenous vaccines market was valued over US$ 116 Mn in 2020, and is predicted to witness an impressive CAGR of 5.5% over the forecast period (2021-2031).
Get Sample Copy of Report @ https://www.persistencemarketresearch.com/samples/29491
Companies covered in Autogenous Vaccines Market Report
- Newport Laboratories, Inc. (Boehringer Ingelheim International GmbH)
- Phibro Animal Health
- Elanco Animal Health
- Ceva Santé Animale
- ACE Laboratory Services (Apiam Animal Health)
- Huvepharma, Inc.
- AniCon Labor GmbH
- Cambridge Technologies
- Dyntec s. r. o
- Hygieia Biological Laboratories
- HIPRA
- Vaxxinova
- sanphar (ipeve)
- Addison Biological Laboratory
- Zoetis (PHARMAQ AS)
- Barramundi Asia Pte Ltd.(UVAXX Asia)
- Kennebec River Biosciences
Get A Customized Scope To Match Your Need Ask An Expert – sales@persistencemarketresearch.com
Get To Know Methodology of Report @ https://www.persistencemarketresearch.com/methodology/29491
Key Takeaways from Market Study
- By type of strain, bacterial autogenous vaccines are expected to contribute to more than 1/3 revenue share in the market.
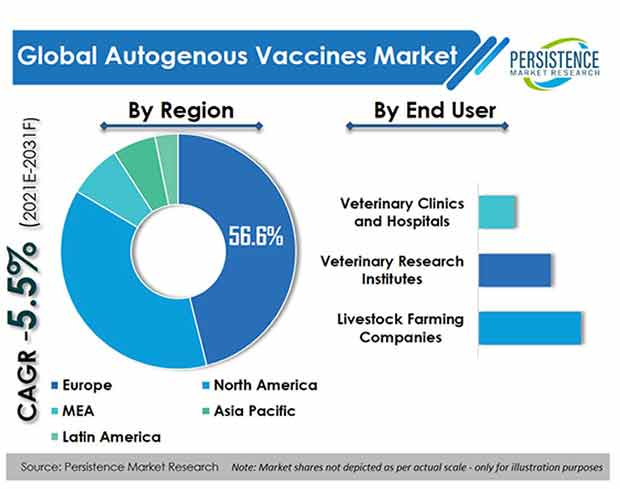
- By end user, livestock farming companies will lead by acquiring over 45% of market share. This sector has witnessed the most adoption of autogenous vaccines as compared to other end users.
- Europe holds around half of the global market share.
- Key players are focused on expanding their product portfolios and expansion at regional levels through collaborations and acquisitions.
- COVID-19 outbreak in big economies is expected to negatively impact demand for autogenous vaccines owing to reduced healthcare center visits. Impact was also observed on vaccine production and transportation owing to travel restriction and lack of workforce at production centers.
- The market in Thailand is projected to be the fastest-growing in the Asia Pacific region, and will expand at a CAGR above 5% through 2031.
- The U.S. market holds a big portion of revenue, and its market share accounts for more than 90% in North America.
“Increasing preference for autogenous vaccines over antibiotics will catalyze market growth over the coming years,” says an analyst of Persistence Market Research.
Access Full Report @ https://www.persistencemarketresearch.com/checkout/29491
Collaborations & Acquisitions Key Strategies amongst Market Players
Prominent players manufacturing autogenous vaccines are collectively increasing their product ranges through strategic initiatives such as acquisitions and reaching out to emerging markets. Increasing investments and manufacturing capacity expansion are expected to favour market growth over the forecast period.
For instance,
- In September 2019, Agri Labs (Huvepharma, Inc.) established a new fermentation plant in Peshtera, Southern Bulgaria.
- In May 2017, Elanco Animal Health opened a new manufacturing facility for autogenous poultry vaccines in Winslow, Maine.
- Agreement to acquire Bayer AG‘s animal health business by Elanco Animal Health in August 2019 is one of the major acquisitions in this space. This strategic move will help Elanco Animal Health enhance its market position.
What Does the Report Cover?
Persistence Market Research offers a unique perspective and actionable insights on the autogenous vaccines market in its latest study, presenting historical demand assessment of 2016-2020 and projections for 2021-2031, on the basis of strain (bacterial strain and viral strain) and end user (veterinary research institutes, livestock farming companies, and veterinary clinics and hospitals), across the five key regions of the world.
Access Related Reports:
E-Clinical Solution Software Market:
According to a new market report published by Persistence Market Research “Global Market Study on E-Clinical Solution Software: Asia to Witness Highest Growth by 2020”, the global e-clinical solution software market was valued at US$ 3,005 Mn in 2014 and is expected to expand at a CAGR of 13.8% from 2014 to 2020, to reach US$ 6,515.3 Mn by 2020.
About us: Persistence Market Research
Contact us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com