The Oral Solid Dosage Contract Manufacturing Market is ascertained to make greater strides in the future. The present-day and futuristic cutting-edge technology, namely IoT, AI, and Big Data operate better in a lightning-fast and reliable internet connection. The benefits of high-speed internet would be seen in telecare in the next 2-3 years, but going forward, more authentic data streams are likely to come up with better-connected devices, thereby revolutionizing the healthcare system.
The Oral Solid Dosage Contract Manufacturing Market Share is expected to be worth US$ 7,201.1 Million at a CAGR of 5.8% between 2028. With value-based reimbursement systems taking the center stage, patient engagement technology is expected to be adopted all across. The pricing analysis takes into consideration licensing fees, implementation fees, annual license maintenance fees, up-gradation and integration fees, and consulting fees.
Market consolidation strategies to be centered around acquisition of key technologies and specialty pharma companies.
Solid dosage continues to remain the most preferred drug delivery form, used across a wide spectrum of pharmaceutical landscape, pushing the global oral solid dosage (OSD) contract manufacturing market to a promising US$ 21.5 Billion cap, in 2019, predicts Persistence Market Research, in its recently released study on the global oral solid dosage contract manufacturing landscape.
Get Sample Copy of Report @ https://www.persistencemarketresearch.com/samples/25768
Company Profiles
- Recipharm AB
- AbbVie Contract Manufacturing
- Patheon N.V.
- Catalent Inc.
- NextPharma
- Capsugel (Lonza Group AG)
- Aurobindo Pharma Limited (AuroSource)
- Siegfried AG
- Piramal Pharma Solutions
- CordenPharma
- Others.
Get A Customized Scope To Match Your Need Ask An Expert – sales@persistencemarketresearch.com
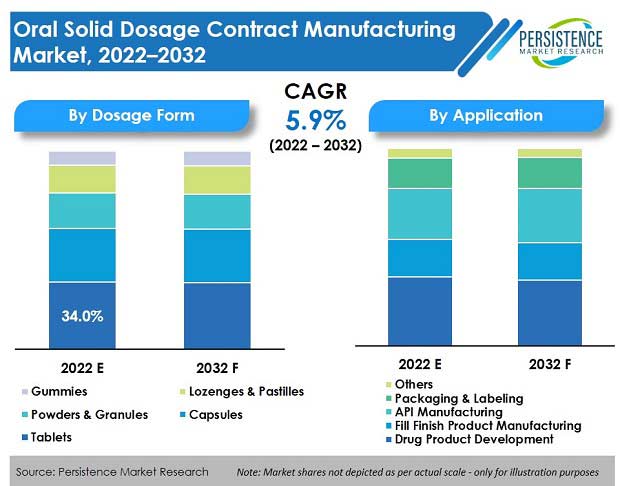
Apart from being more cost-effective (compared to the novel treatment alternatives and increasing number of biologics) and patient complaint, oral dosage provides increased physical and chemical stability, controlled-release options, and superior ease of handling.
The global oral solid dosage contract manufacturing market is estimated to grow at a high CAGR of 5.8 percent, through the forecast period of 2017-2028.
“The market will continue to perform immensely well, given the unrivaled demand for generics, in the coming years. Key companies operating in the oral solid dosage contract manufacturing landscape, anticipate substantial market growth through 2028, given the growing demand in emerging markets, continuing novel pharmaceutical formulations, and industry consolidation”, Principal Analyst, Pharmaceutical Domain, Persistence Market Research
Get To Know Methodology of Report @ https://www.persistencemarketresearch.com/methodology/25768
The global market for oral solid dosage contract manufacturing presents a highly fragmented landscape, wherein approximately 11 percent tier-1 leaders including Patheon N.V., AbbVie Inc., Catalent Inc., Piramal Enterprises, Capsugel (Lonza), and AuroSource hold a lion’s share of nearly 63 percent. In addition, tier-2 and tier-3 players contribute approximately 12 percent and 25 percent to the total market share, respectively. Their key forward market strategies include,
- Increased research and development activities by US-based pharmaceutical companies translating into a number of mergers—aimed at scaling production capacity of New Chemical Entities (NCEs).
- Innovator companies outsourcing active pharmaceutical ingredient (API) and formulation manufacturing is resulting in nearly 60 percent cost cutting.
- Long-term contract manufacturing partnerships between companies, is expected to remain a critical demand growth strategy—with most CMOs building contract manufacturing facilities for pharmaceutical companies. The trend is mostly prevalent in the North American oral solid dosage contract manufacturing landscape
- Product differentiation, in a fragmented landscape, would help companies to solidify their market position in oral solid dosage contract manufacturing market, globally.
- Consolidation remains a perennial issue for the CMO and contract development and manufacturing organization (CDMO), wherein acquisitions of either a standing provider or an existing pharmaceutical facility will dominate the oral solid dosage contract manufacturing landscape, specifically in US.
- Big pharma are projected to balance their product portfolios and make substantial investments in their core capabilities.
- CMOs are likely to strive hard to keep up with regulatory compliances—posing both as a challenge and opportunity for them.
- Partnerships with third-party distributors will remain a highly adopted model.
“In the global oral solid dosage contract manufacturing landscape, approximately 64 percent contract manufacturing organizations (CMOs) pursue contract manufacturing as their primary business.
The remaining 36 percent offer contract manufacturing in production facilities where they manufacture their own products. Of 280 largest CDMOs only 12.5 percent operate on global level i.e. their manufacturing and business development activities cover multiple geographical regions. Majority of medium to small size CMOs focus to serve their immediate markets”, the principal analyst explained further.
Novel technologies in the form of sublingual tablets and extended release beads in capsules are anticipated to benefit OSD contract manufacturers to either introduce new products or revive their older products.
Apart from catering well to the patient segment having difficulties in swallowing tablets, including pediatric patients, extended release beads help in optimizing release rate, while reducing initial dose spike. Sublingual tablets delivers quick medication, mostly used in pain management applications. Innovators are also betting on Amorphous solid dispersions (ASDs), providing them with more efficient solubility and stability, translating into safer drugs with enhanced efficacy.
Access Full Report @ https://www.persistencemarketresearch.com/checkout/25768
Insights from Segmentation Analysis of Oral Solid Dosage (OSD) Contract Manufacturing Market
- Tablet remain the most commonly preferred dosage form given the growing demand for specialized dosage forms including pediatric tablets and orally disintegrating tablets. By dosage form, tablets are expected to generate a revenue of US$ 11.2 Bn, in 2019, with immediate tablets contributing the maximum revenue share.
- By end-user, small & medium size pharma and biotech companies will remain the most lucrative customers, in terms of revenue generation for oral solid dosage (OSD) contract manufacturing, considering small & medium pharma companies lack full-potential manufacturing facilities.
About us: Persistence Market Research
Contact us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com