The Immune Checkpoint Inhibitors Market is expected to be worth US$ 35 Bn by the year 2028, at a CAGR of 14.6% between 2018-2028. Digital health is into occupation of a noteworthy position in our lives, thereby rendering it a significant tool in the medical device trends going forward. This would be the overall outlook of the healthcare vertical in the forecast period.
Attributed to growing demand for the development of biologics targeting cancer therapy, PMR predicts that the immune checkpoint inhibitors will present lucrative opportunities to investors in the near future – as quoted by a research expert at Persistence Market Research (Healthcare & Life Sciences).
Get Going With Sample Of Immune Checkpoint Inhibitors Market Report! https://www.persistencemarketresearch.com/samples/20122
Expanding at a booming CAGR of 14.6%, the global market for immune checkpoint inhibitors market is expected to reach a value beyond US$ 35 Bn over 2018-2026. The growing prevalence of various types of cancers and expanding healthcare expenditure will remain the most prominent drivers of the immune checkpoint inhibitors market over the forecast period.
Company Profiles:
- AstraZeneca Plc.
- Bristol-Myers Squibb Co.
- Roche Holding AG
- Incyte Corporation
- Merck & Co., Inc.
- Merck KGaA
- Sanofi
- Novartis AG
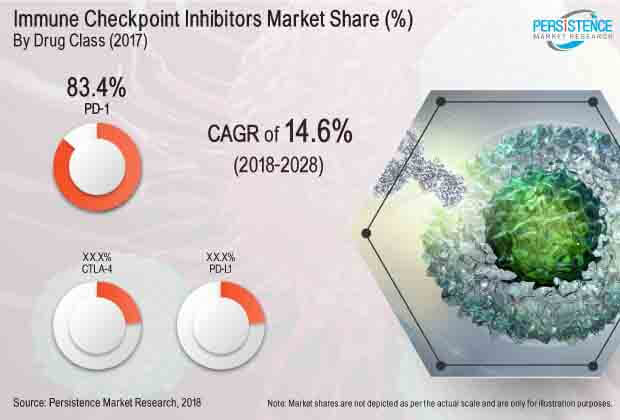
Besides cancer prevalence, increasing FDA approvals and growing use of immune-oncology products in treating cancers will also push the demand for immune checkpoint inhibitors in the near future. However, high price point associated with both drug development and treatment will continue to restrict adoption. Frequently observed last stage failure will also remain a major concern in the long run.
“Our extensive company share analysis predicts that the top two players in the global immune checkpoint inhibitors market currently hold a massive revenue share of over 95%. Based on the exhaustive assessment of each drug class in the global immune checkpoint inhibitors market, we arrived at the forecast that the PD-1 will continue dominance in the market, primarily attributed to rapid FDA approvals to PD-1 products,” explained an expert senior research analyst working with the healthcare and life sciences domain at Persistence Market Research.
How About Re-Inventing The Methodical Wheel In The Immune Checkpoint Inhibitors Market? Switch Over To The “Methodology” Tab! https://www.persistencemarketresearch.com/methodology/20122
FDA Approvals Continue to Encourage North American Market for Immune Checkpoint Inhibitors
North America is likely to continue monopoly in the global market for immune checkpoint inhibitors with over 65% share of the total market revenue. Large number of FDA approvals, expanding application base in treatments for various cancer types, and increasing combination drug approvals will play a pivotal role in shaping the market for immune checkpoint inhibitors in North America.
According to PMR’s regional analysis of global immune checkpoint inhibitors market, Asia Pacific will demonstrate vigorous growth throughout the projection period – at the double digit CAGR of over 17%.
High Potential Combination Therapies to Open New Doors of Opportunities
With a strong reference post success of the promising results delivered by Bristol-Myers Squibb using a combination of Yervoy and Opdivo, the market for immune checkpoint inhibitors has been witnessing several more combination therapy products in the pipeline.
Such a scenario is responsible for the current situation of the immune checkpoint inhibitors marketplace that reflects a major paradigm shift of companies from conventional mono-therapies to combination therapies for better end results. While a considerable number of combination therapies introduced by various companies have been successfully contributing to the efforts in transforming oncology, the global market for immune checkpoint expects successful introduction of more such therapies.
One of those in the pipeline includes the combination with target specific mAbs, chemotherapy, and other checkpoint inhibitors.
Keeping A Tab On Key Players In The Immune Checkpoint Inhibitors Market? Go To “Purchase Now” To Decipher The Competitive Analysis In Our Immune Checkpoint Inhibitors Market Report! https://www.persistencemarketresearch.com/checkout/20122
Key Players to Invest Efforts in Developing Products to Traverse Diverse Indications
The global immune checkpoint inhibitors market is competitive yet consolidated due to strong presence of established players; however, a few prominent brands such as Tecentriq have strategically maintained a consistent position in the market since their first product launch. A majority of leading players participating in the global immune checkpoint inhibitors marketplace are concentrating on incorporating value addition programs for regulating the customer base through enhancement of product offerings to suit a diverse range of indications.
Established companies in the global immune checkpoint inhibitors market landscape are striving to enhance therapeutic applications of their existing product offerings. Moreover, a number of key players are also eyeing the high potential markets emerging in developing countries such as KSA and South Africa by seeking FDA approvals to their products in these markets.
About Us: Persistence Market Research
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com