The Urothelial Carcinoma Diagnostics Market is expected to grow on a persistent note in the future. With AI making its mark everywhere, how could the healthcare vertical be left behind? Abnormalities in the medical scans could be easily spotted by radiologists with the help of machine learning. Likewise, the healthcare vertical is poised to develop on a ravishing note with technological advancements on the anvil in the next decade.
According to World Health Organization (WHO) estimates, every year, 3.8 Mn new cases of urothelial carcinoma occur around the world, with the recurrence rate being nearly 30-50%. However, early detection by use of modern diagnostics enables quick medical decision-making and consequent treatment for urothelial carcinoma. Accuracy in urothelial carcinoma diagnostics plays a significant role in the reduction of treatment costs.
Moreover, increased demand for treatment of urothelial carcinoma is a key driver for increasing revenue inflow from diagnostic tests and procedures. However, development of effective urothelial carcinoma screening methods will play a significant role in the early detection and subsequent reduction in mortality rates. Furthermore, technological advancements in diagnostic methods such as the adoption of diagnostic imaging procedures, rapid molecular diagnostic kits, and use of biomarker testing in urothelial carcinoma screening programs are some other factors that will aid the growth of the urothelial carcinoma diagnostics market.
To remain ‘ahead’ of your competitors, request for a sample @ https://www.persistencemarketresearch.com/samples/14128
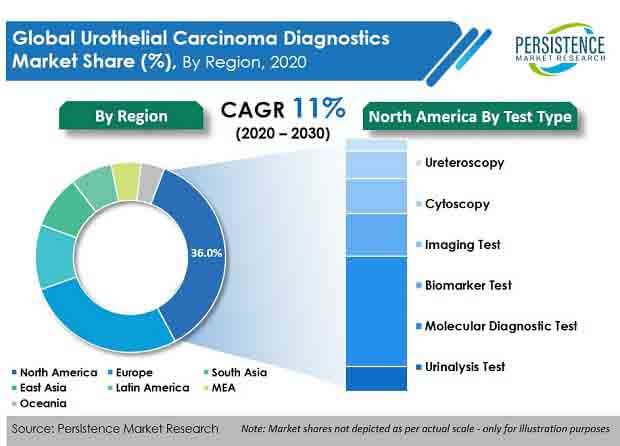
According to the latest report published by PMR, the global urothelial carcinoma diagnostics market was valued at US$ 1 Bn in 2020, and is expected to progress at a CAGR of over 11% during the forecast period (2020–2030).
Key Takeaways from Urothelial Carcinoma Diagnostics Market Study
- The reagent and kits segment, under product type, is expected to contribute more than 70% of revenue share in the urothelial carcinoma diagnostics market.
- By test type, molecular diagnostic tests accounted for the highest revenue in 2019. Rapid and sensitive detection is anticipated to drive demand for urothelial carcinoma diagnostics.
- Based on end user, the hospitals segment accounts for a major revenue share in the urothelial carcinoma diagnostics market.
- Leading players launching rapid diagnostic products with advances technology are expected to dominate the urothelial carcinoma diagnostics market space.
- Governments of various countries are organizing cancer screening programs and are spreading awareness, which is creating significant demand for urothelial carcinoma diagnostics.
- The COVID-19 outbreak has resulted in manufacturing disruptions and also decreased demand, which is projected to impede market growth in the near term.
Company Profiles:
- GE Healthcare
- Roche Holding
- Illumina
- IDL Biotech
- Agilent Technologies
- Olympus Corporation
- Philips Healthcare
- Abbott Molecular
- Bio-Rad Laboratories, Inc
- Thermo Fisher Scientific, Inc.( Qiagen N.V.)
- Danaher Corporation (Cepheid)
Get a Customized Scope to Match Your Need Ask an Expert- sales@persistencemarketresearch.com
“Rise in prevalence of urothelial carcinoma and demand for early detection for preventive treatments will boost the global urothelial carcinoma diagnostics market,” says a PMR analyst.
Acquisitions and Partnerships – Key Strategies amongst Market Players
Key players in the urothelial carcinoma diagnostics market are looking forward to strengthening their product portfolios through the launch of new products. For instance, in 2017, Roche received FDA approval for the complementary PD-L1 (SP263) biomarker test in urothelial carcinoma. In August, 2018, Agilent Technologies, Inc announced that the US Food and Drug Administration approved its Dako PD-L1 IHC 22C3 pharmDx assay for expanded use as a companion diagnostic test for Merck’s anti-PD1 immunotherapy Keytruda (pembrolizumab) for urothelial carcinoma.
For in-depth competitive analysis, buy now @ https://www.persistencemarketresearch.com/checkout/14128
Read More Trending “PMR Exclusive Article”-
Treponema Pallidum Tests Market–Treponema Pallidum Tests Market Segmented By Treponema Pallidum Particle Agglutination Assay Test, Fluorescent Treponemal Antibody Absorption Test, Treponema Pallidum Hemagglutination Assay Treponemal test and Venereal Disease Research Laboratory Test, Rapid Plasma Reagin Test, Toluidine Red Unheated Serum Test Non-treponemal tests
About Us: – Persistence Market Research
Contact us:
Persistence Market Research
Address – 305 Broadway, 7th Floor New York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com
Website – https://www.persistencemarketresearch.com