The Biologics Contract Manufacturing Demand Market is likely to mark stellar growth in the upcoming period. The current situation calls for conducive integrated healthcare models that do away with middlemen regarding offering various products and services. Drug manufacturers, hospital systems, PBMs (pharmacy benefit managers), wholesale distributors, hospital systems, medical insurers, and retail pharmacy outlets – are all into the integration of healthcare strategies. This would be how the healthcare vertical function in the upcoming period.
Pharmaceuticals and biotechnology companies are outsourcing specific services from the early stages of drug development to biological contract manufacturing organizations to reduce drug manufacturing costs. Outsourcing increases drug manufacturing efficiency and facilitates flexible operational capabilities, which is expected to immensely boost biologics contract manufacturing demand. Rising prevalence of chronic and lifestyle diseases is also anticipated to propel biologics contract manufacturing demand. Besides, continuous focus by manufacturers toward adopting technologically-advanced systems to manufacture novel biologics is also expected to accelerate the demand for biologics contract manufacturing over the forecast period.
To remain ‘ahead’ of your competitors, request for a sample @ https://www.persistencemarketresearch.com/samples/31928
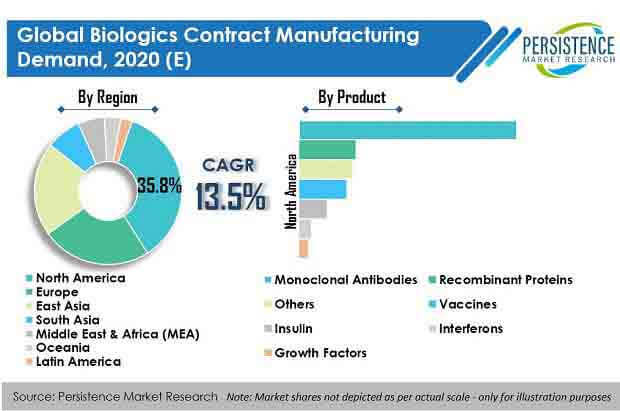
Global biologics contract manufacturing demand is expected to reach at 8 Mn liters (MnL) in 2030, exhibiting an impressive CAGR of over 17% during the forecast period (2020 – 2030).
Key Takeaways from Biologics Contract Manufacturing Demand Study
- Leading global CDMOs are focusing on expansion of their manufacturing capabilities with high flexibility and rapid operation capacity as biologics contract manufacturing demand is high. With the help of advanced single-use technologies, companies are able to cut batch manufacturing and cleaning time from 7 days to 1 day. This trend is expected to provide a competitive-edge to companies in terms of capacity, cost, convenience, and operational efficacy.
- For instance, in Oct 2018, Thermo Fisher Scientific Inc. announced a US$ 50 million (€44 million) expansion at a CDMO plant in St Louis, Missouri, U.S., to add 16,000 L of single-use capacity due to extremely strong growth in demand for commercial biologic manufacturing. As per the company, the facility will be the largest outsourced single-use biologics site in North America.
- With advancements in contract solutions, leading CDMOs are focusing on the expansion of their integrated capabilities from molecule-to-finished product. This trend is significant as the acceleration of development and speed to market is becoming more crucial for biopharmaceutical therapeutic developers.
- For example, in July 2020, Avid Bioservices and Argonaut Manufacturing Services entered into an agreement to offer biotechnology and pharmaceutical clients integrated process development, drug substance manufacturing, and drug product parenteral manufacturing, to accelerate the development and commercialization of biopharmaceutical therapeutics.
- Viral vector and vaccines contract development and manufacturing services are expected to gain significant traction due to the COVID-19 pandemic breakout and subsequent vaccine development programs.
- For instance, in June 2020, AGC Biologics expanded its development capacities for pDNA services at the Heidelberg site, Germany, to meet the growing need for process development and manufacturing for plasmid DNA (pDNA).
- Europe is expected to be the most lucrative region and with a revenue share of around one-third in global biologics contract manufacturing demand in 2020, owing to the presence of leading CDMO facilities in the region.
“Technological advancements to improve the manufacturing efficiency of biologics at low cost is expected to flourish market growth opportunities and provide a competitive advantage to market players in terms of innovation,” says a PMR analyst.
Company Profiles:
- BioXcellence (Boehringer Ingelheim GmbH)
- Lonza Group AG
- Samsung Biologics Co. Ltd.
- Fujifilm Diosynth Biotechnologies (FUJIFILM Holdings)
- AbbVie Contract Manufacturing (AbbVie Inc.)
- WuXi Biologics (Cayman) Inc.
Get a Customized Scope to Match Your Need Ask an Expert- sales@persistencemarketresearch.com
Doubling Industry Capacity by 2022-2023 amid COVID-19 Pandemic
The COVID-19 pandemic is becoming an industry changing milestone by providing opportunities to global CDMOs – small as well big CDMO companies. Local government and industry funds are being targeted toward rapid expansion of industry capacity in order to meet the unmet demand for vaccine development and commercialization amid the COVID-19 crisis.
For instance, in June 2020, Fujifilm Diosynth Biotechnologies announced the investment of 100 billion Yen (US$ 928 Million) to expand its large-scale biologics production facility in Denmark.
Expansion of Manufacturing Capacity – Imperative Strategy for Market Players
Key players involved in biologics contract manufacturing are looking forward to expand their manufacturing capacities to meet global demand. Leading companies are expanding their production capabilities and capacities to become the preferred choice for innovative biopharmaceutical companies and hold on to a strong position in the market.
For instance, in January 2019, Boehringer Ingelheim Biopharmaceuticals China expanded its commercial manufacturing capacities. The company installed an additional bioreactor, all needed utility, and infrastructure for GMP operations.
What Does the Report Cover?
Persistence Market Research offers a unique perspective and actionable insights on biologics contract manufacturing demand in its latest study, presenting historical demand assessment of 2015 – 2019 and projections for 2020 – 2030, on the basis of platform (mammalian and microbial), application (commercial and clinical) product (monoclonal antibodies, recombinant proteins, vaccines, insulin, interferons, growth factors, and others), and therapeutic area (oncology, autoimmune disease, metabolic disease, ophthalmology, cardiovascular disease, infectious disease, neurology, respiratory disorders, and others), across seven key regions.
For in-depth competitive analysis, buy now @ https://www.persistencemarketresearch.com/checkout/31928
About Us: – Persistence Market Research
Contact us:
Persistence Market Research
Address – 305 Broadway, 7th Floor New York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com
Website – https://www.persistencemarketresearch.com