The Pyrogen Testing Market is expected to grow on an irrevocable note in the upcoming period. The medical landscape is witnessing an influx of at-home diagnostic kits. This trend of on-demand products is likely to take the healthcare vertical by storm in the upcoming period. It has been observed that home kits aid in educating patients and bringing them to the medical visits better prepared, thereby curtailing the time taken for diagnosis. The status quo would help in keeping the healthcare vertical’s cash registers ringing going forward as well.
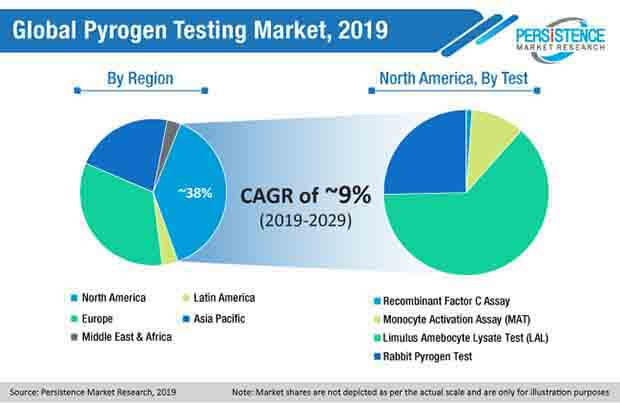
The new market intelligence report by Persistence Market Research (PMR) reveals that the global pyrogen testing market will reach US$ ~800 Mn by the end of 2019, and further exhibit a robust CAGR of 9% through the next decade. The increasing trend of outsourcing drug manufacturing to low-cost production regions by most pharmaceutical companies has been touted to remain a major influencer associated with the growth of pyrogen testing market landscape over the period of assessment, as it allows for low-cost skilled workforce and ease in tax relaxation.
Get Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/4259
Company Profiles:
- Thermo Fisher Scientific, Inc.
- Merck & Co., Inc.
- Associates of Cape Cod, Inc.
- Charles River Laboratories, Inc.
- GenScript
- Lonza
- Hyglos GmbH
- Wako Chemicals USA, Inc.
- Microcoat Biotechnologie GmbH
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/4259
Growing Number of Pharmaceutical and Biotechnology Companies to Bring Traction to Pyrogen Testing
As medical devices cross the barriers within the body leading to contamination, pyrogen testing could be used by healthcare professionals to detect the harmful substances present in those devices. In addition, growing healthcare awareness also leads to upsurge in the medicine demand, which increases establishment of pharmaceuticals and biotechnology industries. This in turn, propels the demand for pyrogen testing in these establishments worldwide. Moreover, increase in number of people diagnosed with various diseases results in the rising demand for various injectables, vaccines, tablets, and other medicines in turn, fueling the demand for safety measures such as pyrogen testing.
According to the PMR analysis, in end user, pharmaceutical companies will dominate the demand side in the pyrogen testing market by the end of 2019 and will also continue to exploit the highest share in the forecast period. This is due to the growing number of pharmaceutical establishments in developed as well as developing countries, which increases the need for drug safety checks like pyrogen testing.
Demand for limulus amebocyte lysate (LAL) pyrogen testing is estimated to grow by the end of 2029, as end users demands for user-friendly pyrogen testing method that are cost-effective, and incur low equipment cost as well. Thus, the PMR study report estimates that LAL pyrogen testing method will harness around 60% of the global pyrogen testing market share during the forecast period.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/4259
Favorable Government Initiatives to Augur Well for Pyrogen Testing Market
Increasing spending by government bodies to develop effective drugs and medical devices is expected to drive the growth of the global pyrogen testing market in the forecast period. Pyrogen is a prominent cause that leads to shock or fatality among a large section of the patient population creating an economic burden on the country. Thus, increasing healthcare spending is expected to result in growing demand for pyrogen testing by various pharmaceutical and medical device companies. Also, government in various countries are focused on prevention of infectious diseases to reduce associated healthcare costs, which in turn is expected to result in high demand for healthcare facilities.
In order to further complement this, government is focusing on pyrogen testing policies for contaminant-free medical devices and pharmaceuticals provided to the patient population, which will pave the way for the elevated requirement of pyrogen testing in the market. Hence, companies operating in the pyrogen testing market are expected to largely benefit from such developments.
PMR’s analysis also projects groundbreaking perspectives on the competitive business scenario of the pyrogen testing market along with highlights of participants’ key business policies and approaches. For instance, many drug manufacturing units are investing in foreign nations, especially in emerging economies of Asia Pacific and Latin America, which hold great future opportunities for the pyrogen testing market. Various pharmaceutical and medical device manufacturers are establishing their manufacturing base in India, China, and others in order to tap and cater to the unmet needs of the patient population in these regions, thus escalating the market for pyrogen testing.
About us: Persistence Market Research
Contact us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com