The Newborn Metabolic Screening Market is bound to reach 1.5X at a CAGR of 9% between 2020 – 2030. The current scenario is such that on-demand healthcare storage is being asked for. Cloud computing thus curtails operational expenses and capital as it simplifies sharing medical records, creates and maintains telehealth apps, and automates backend operations. This would be the scene with the healthcare vertical in the upcoming period.
Increasing birth rates across the world are expected to drive demand for newborn metabolic screening, as the risk of galactosemia, sickle cell disease, phenylketonuria, maple syrup urine disease, and cystic fibrosis is higher among infants. Various countries are implementing screening for metabolic diseases in newborns as a part of their immunization programs. To remain competitive and ensure a robust product pipeline, leading companies continue to identify potential assets through pilot screening programs, which typically have limited resources to see projects through the market.
Get Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/31998
According to a latest report published by Persistence Market Research, the global newborn metabolic screening market was valued at around US$ 247 Mn in 2020, and is expected to witness an impressive CAGR of over 9% during the forecast period (2020 – 2030).
Company Profiles:
- PerkinElmer
- Bio-Rad Laboratories
- Trivitron Healthcare Private Limited
- BioMedomics, Inc.
- Luminex Corporation
- MP BIOMEDICALS
- Synergy Medical Systems LLP
- Agilent Technologies, Inc
- Thermo Fisher Scientific, Inc
- Zivak Technologies
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/31998
Key Takeaways from Market Study
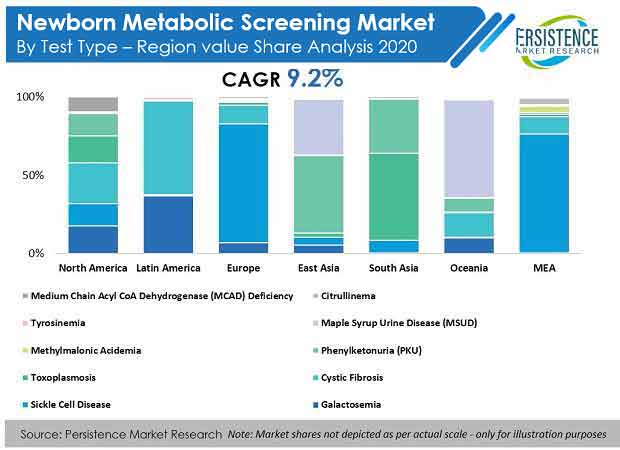
- Sickle cell disease is expected to contribute more than 30% of revenue share to the market.
- By sample, blood testing held the highest market share in 2019 due to efficient diagnostic results.
- Europe holds a significant share of over 30% in the global newborn metabolic screening market.
- Leading manufacturers are focused on strengthening their product portfolios and regional expansion through collaborations.
- Hospitals are expected to hold significant market share, due to high number of screening programs being arranged in collaboration with hospitals.
- The COVID-19 outbreak has had a negative impact on the market, as short-term lockdowns disturbed the production and supply chain of kits.
- The market in the U.S is projected to expand at a CAGR of close to 10% through 2030.
- The U.K market has a share of more than 17% in Europe, and is expected to progress at a CAGR of more than 12% over the next ten years.
“Increasing prevalence of new born metabolic diseases and rising adoption of related screening program will boost the global market,” says an analyst of Persistence Market Research.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/31998
Collaborations & Acquisitions Key Strategies amongst Market Players
Leading players in the newborn metabolic screening landscape are focusing on improving their product portfolios through partnerships and acquisitions. Various players are also focusing on regulatory approvals and innovative product launches.
- On October 05, 2020, PerkinElmer announced CE-IVD approval of the EONIS™ newborn screening assay.
- In October 2018, Trivitron’s Labsystems Diagnostics launched high quality newborn screening panels.
- On September 3, 2020, Sickle SCAN® was approved by the Department of Health in Australia.
What Does the Report Cover?
Persistence Market Research offers a unique perspective and actionable insights on the newborn metabolic screening market in its latest study, presenting historical demand assessment of 2015 – 2019 and projections for 2020 – 2030, on the basis of test (galactosemia, sickle cell disease, cystic fibrosis, toxoplasmosis, phenylketonuria (PKU), methylmalonic acidemia, maple syrup urine disease (MSUD), tyrosinemia, citrullinemia, and Medium Chain Acyl CoA Dehydrogenase (MCAD) Deficiency), sample (blood and urine), and end user (diagnostic laboratories, specialty clinics, and hospitals), across seven key regions of the world.
About us: Persistence Market Research
Contact us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com