The global market for temporary cardiac pacemaker leads is estimated to grow at a rate of 6.6% in the coming period, reaching US$411.5 million by 2026. Integration with virtual reality solutions is expected to rule in the forecast period. Augmented reality could be used to access information as well as reports while seeing patients or without having to leave your existing operations, that too, in a completely hands-free mode, through voice command or by doing support data to appear automatically. This would be the vertical of healthcare in the future.
The prevalence of arrhythmias and cardiovascular diseases is increasing and is expected to continue to grow during the forecast period. According to the company’s latest research, the global market for temporary cardiac pacemaker leads and leads is anticipated to account for more than US$411.5Mn in value terms by the end of 2026 . The report on the market for temporary cardiac pacemaker leads and leads indicates that the market is anticipated to have significant growth potential until 2022.
Get a free sample copy of the report @ https://www.persistencemarketresearch.com/samples/25129
Minimally invasive procedure for transvenous pacing, by technique type, is anticipated to account for the largest share of revenue during the forecast period. The increasing adoption of minimally invasive technique for temporary pacing during emergencies such as acute myocardial infarction and heart block is expected to boost the market for temporary cardiac pacing leads and leads in the near future.
company profiles
- Medtronic plc.
- BioTrace Medical Inc.
- Becton, Dickinson and Company (CR Bard)
- B. Braun Melsungen AG
- Abbott Laboratories (St. Jude Medical)
- Edwards Lifesciences Corporation
- built-in teleflex
- OSCOR inc.
- A&E Medical Corporation
- OSYPKA AG
- Others.
The favorable reimbursement scenario for the treatment of cardiovascular diseases, as the burden of cardiovascular diseases continues to increase in developing and underdeveloped economies, contributes to the growth of the market for temporary cardiac pacemaker leads and leads. Significant growth in the number of cardiac surgeries is also driving the market for temporary cardiac pacemaker leads and leads.
According to the Centers for Disease Control and Prevention (CDC), in the United States, about 735,000 Americans experience heart attacks each year. An estimated 2.7 to 6.1 million people in the United States have atrial fibrillation, which is expected to increase further as the US population ages, with more than 750,000 hospitalizations each year due to atrial fibrillation. atrial fibrillation.
Learn about the report methodology @ https://www.persistencemarketresearch.com/methodology/25129
The increasing number of cardiac surgeries, primarily among the geriatric population, coupled with the anticipated increase in the number of people undergoing cardiothoracic surgeries, is expected to drive the market for temporary cardiac pacemaker leads and leads during the forecast period.
The rapid aging of the population also contributes to the significant growth in demand for temporary pacing leads and leads, leading to the growth of the temporary pacing leads and leads market.
According to the Population Reference Bureau (2016), the number of Americans age 65 and older is projected to more than double, from 46 million to more than 98 million, by 2060 and the share of the age 65 and older group in the population. the total population will increase from 15% to almost 24%.
Technological advances in cardiac devices are also driving the growth of the temporary cardiac pacemaker leads and leads market. The increasing demand for pacemakers, due to the increasing prevalence of heart diseases, mainly due to the increase in obesity, unhealthy lifestyles, smoking, drug abuse and excessive alcohol consumption among the young population, is causing various cardiac disorders such as bradycardia and heart block. .
This is expected to further increase the demand for temporary cardiac pacemaker leads and leads during the forecast period.
Manufacturing companies in the market for temporary cardiac pacemaker leads and leads are focusing on the development of high-tech devices and further aiming towards launching devices used in minimally invasive techniques.
The launch of new products embedded with advanced technologies that facilitate treatment procedures and provide better outcomes for patients is also contributing to the growth of the market for temporary cardiac pacemaker leads and leads.
Access the full report @ https://www.persistencemarketresearch.com/checkout/25129
For example, in October 2016, BioTrace Medical, Inc. received 510(k) clearance from the US Food and Drug Administration to market a new Tempo Lead, an innovative temporary pacemaker lead designed for use in procedures where temporary pacing is indicated, including transcatheter aortic valve replacement (TAVR) and electrophysiology (EP) procedures.
Increasing demand for cardiovascular surgeries is expected to contribute to leads and wires sales and in turn contribute to the growth of the temporary cardiac pacemaker leads and wires market.
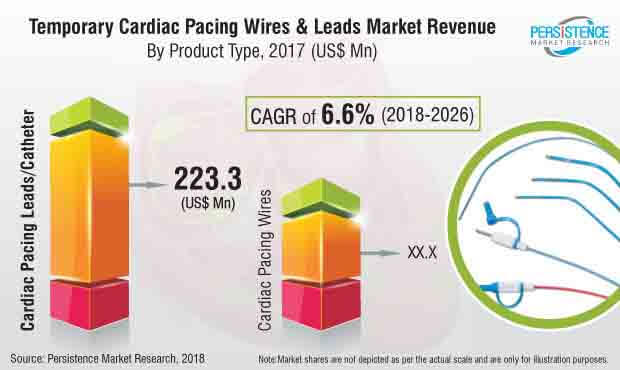
The global Temporary Cardiac Pacemaker Leads and Wires market has been segmented on the basis of product type, application, technique, age group, and end user. In terms of revenue, the cardiac stimulation leads/catheters segment by product type in the temporary cardiac stimulation leads and wires market is expected to hold a significant share during the forecast period.
About us: Persistence Market Research
Contact us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com