The global Lateral Flow Assays Market is bound to witness a CAGR worth satiating In Upcoming Years. In the era of cloud computing, the cloud revolution is there to break the stereotypes. Several key stakeholders are going for cloud hosting solutions providers to enhance their accounting services. This migration to cloud technology is making way for the enterprises to simplify their daily tasks, that too, conveniently and cost-effectively. As such, the industry could be on the “cloud computing nine” in the forecast period.
As per Persistence Market Research’s latest industry analysis, the global lateral flow assay market is expected to witness high growth during the forecast period, from US$ 3.7 Bn in 2020 to over US$ 6.4 Bn by 2031. This reflects a CAGR of around 5.1% over the forecast period (2021-2031).
Get Free Sample Copy Of Lateral Flow Assays Market Report@ https://www.persistencemarketresearch.com/samples/18613
Lateral flow assays are mainly used for diagnostics for home testing, laboratory use, and point-of-care testing. The most widely applied and well-known applications of lateral flow assays are home pregnancy tests. Demand for lateral flow assays is increasing due to growing adoption of home-based lateral flow assay kits, demand growth of point-of-care testing, and rise in adoption of LFA kits due to their advantages over laboratory tests.
Rising prevalence of infectious diseases is a prime driver for the market. Emergence of diseases such as coronavirus, Zika virus, Ebola, and others has increased the procurement of point-of-care testing devices and kits by international bodies such as UNICEF. Furthermore, the current COVID-19 pandemic has boosted demand for lateral flow tests. COVID-19 infections are diagnosed with lateral flow assay-based tests across the world.
Mobile phones provide rapid results in real time, saving time and reducing result reporting delays. They also allow users to record the time and results of a test. Further, easy and cost-effective sharing of data along with cloud integration has resulted in better centralization and analysis of data using online information sharing platforms. This technology enables clinicians or patients in remote areas to image samples that can be used for TB and malaria diagnosis.
- In January 2021, Sensyne Health, based in the United Kingdom, released MagnifEye, a revolutionary smartphone software that uses deep machine learning and AI to automate the accurate interpretation of lateral flow diagnostic tests.
Company Profiles:
- Abbott Laboratories
- bioMerieux S.A
- Bio-Rad Laboratories Inc.
- Danaher Corporation
- QIAGEN N.V.
- Thermo Fisher Scientific, Inc.
- Siemens Healthineers
- Hologic Inc.
- F. Hoffmann-La Roche Ltd
Request for Methodology@https://www.persistencemarketresearch.com/methodology/18613
Key Takeaways from Market Study
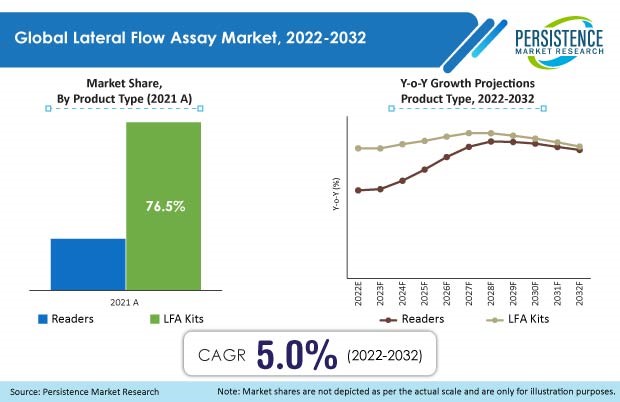
- By product, LFA kits will hold a high share of 70% in 2021, expanding at a mid-single digit CAGR during the forecast period. Primary factors that support rising adoption of products in this segment include ease of use, low cost, and ease of production.
- By indication, infectious disease is expected to hold well over 50% market share in 2021, and is expected to continue growing at a moderate rate in correlation with increasing prevalence of infectious diseases globally.
- High adoption of point-of-care testing for quick diagnosis at hospitals has led to the hospital pharmacies hold a high market share.
- By region, North America held nearly 1/3 of the global market share in 2021. This is attributed to rise in health care expenditures, increasing adoption of lateral flow assays, and presence of prominent players in the region.
“Although the COVID-19 pandemic boosted market growth in 2020, it is expected to be a temporary phase. However, developments such as smartphone-based readers for LFA will continue to provide fruitful growth opportunities for market players over the coming years,” says an analyst of Persistence Market Research.
Market Competition
Some of the leading manufacturers of lateral flow assays are focusing on product approvals and launches as the key growth strategy for global expansion, thereby enhancing their market presence.
- In June 2021, Bio-Rad Laboratories announced the release of its PREvalence ddPCR SARS-CoV-2 wastewater quantifying kit, which is an accurate, sensitive, and cost-effective technique for detecting SARS-CoV-2 infection in community wastewater. The test aids a community in determining if the virus has been prevalent for days or weeks before it is discovered among people.
- In August 2020, Abbott announced that the US FDA granted an Emergency Use Authorization (EUA) for its BinaxNOWTM COVID-19 Ag Card fast test for COVID-19 infection detection.
- In August 2017, Beckman Coulter received FDA clearance for the first IVD test delivering flow cytometric leukaemia & lymphoma analysis in routine clinical labs.
Key market players covered by Persistence Market Research include Abbott Laboratories, bioMerieux S.A, Bio-Rad Laboratories Inc., Danaher Corporation, QIAGEN N.V., Thermo Fisher Scientific, Inc., Siemens Healthineers, Hologic Inc., and F. Hoffmann-La Roche Ltd.
Press The “Purchase Now” Button To Have Our Lateral Flow Assays Market Report@ https://www.persistencemarketresearch.com/checkout/18613
What Does the Report Cover?
Persistence Market Research offers a unique perspective and actionable insights on the lateral flow assay market in its latest study, presenting historical demand assessment of 2016 – 2020 and projections for 2021 – 2031.
The research study is based on the product (readers {bench-top readers and hand-held readers}, LFA kits {test strips, dipsticks, cassettes, and lancets}), indication (infectious diseases {mosquito-borne diseases, streptococcus infections, sexually transmitted diseases, hepatitis, tuberculosis, gastrointestinal infections, and others}, pregnancy test, and drug of abuse testing), and distribution channel (hospital pharmacies, retail pharmacies, supermarkets/hypermarkets, and e-Commerce), across five major regions of the world.
About Us:
Persistence Market Research (PMR), as a 3rd-party research organization, does operate through an exclusive amalgamation of market research and data analytics for helping businesses ride high, irrespective of the turbulence faced on the account of financial/natural crunches.
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com