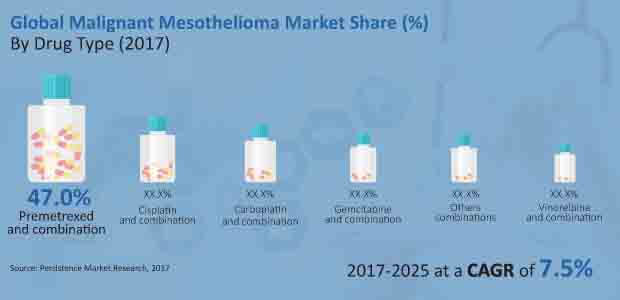
The global Malignant Mesothelioma Market is expected to reach close to US$ 160 Mn, or more than 45% of the market’s total revenue, in 2017 and more than US$ 300 Mn by the end of the forecast period of 2017–2025, growing at a CAGR of 8.4%. The segment by drug type with the highest market share is pemetrexed and combination therapies.
Request for Free Sample Copy@ https://www.persistencemarketresearch.com/samples/10628
Due to the superior response rate of pemetrexed, which ranges from 30% to 40% whether used alone or in combination with other medications, the pemetrexed and combination sector is predicted to increase in market share by more than 300 BPS by 2025 compared to 2017.
In terms of revenue, Pemetrexed and the combination drug type segment dominated the global malignant mesothelioma market in 2016, and this trend is anticipated to continue over the course of the forecast period. The segment for pemetrexed and combinations is also the most appealing, with a market attractiveness score of 3.2 over the anticipated term.
Get up to 20% discount on Full Report Purchase @ https://www.persistencemarketresearch.com/checkout/10628
Market Taxonomy : Malignant Mesothelioma Market
-
- Malignant Mesothelioma Market By Drug Type : Pemetrexed, Cisplatin, Carboplatin, Gemcitabine, Vinorelbine, Others
- Malignant Mesothelioma Market By Route of Administration : Oral, Parenteral
- Malignant Mesothelioma Market By Region : North America, Latin America, Europe, APAC, MEA
- Malignant Mesothelioma Market By Distribution Channel : Hospital Pharmacies, Retail Pharmacies, Oncology Centers
Request For Customization @ https://www.persistencemarketresearch.com/request-customization/10628
Companies Covered in This Report – Malignant Mesothelioma Market
-
- AstraZeneca Plc.
- Bristol-Myers Squibb Company
- Hoffmann-La Roche Ltd.
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi
- Eli Lilly and Company
- Teva Pharmaceuticals
- Boehringer Ingelheim GmbH
- Mylan N.V.
- Fresenius Kabi AG
- Sun Pharmaceuticals Industries Ltd
- Corden Pharma International GmbH
- Concordia International Corp
- Kyowa Hakko Kirin Co Ltd.
- Polaris Pharmaceuticals, Inc.
- MolMed SpA
- Ono Pharmaceutical Co. Ltd
- Nichi-Iko Pharmaceutical Co., Ltd
It is assumed that asbestos exposure leads to malignant mesothelioma and given the restrictions on asbestos use globally, it is expected that cases of mesothelioma will reduce in the near future. However, the number of malignant mesothelioma cases do not support these assumptions. As per statistics released by the Cancer Research UK, 1 in every 140 men and 1 in every 710 women are diagnosed with malignant mesothelioma. It has been observed that there is an increasing trend of malignant mesothelioma cases in men. This particular factor is fuelling the growth in revenue of the Pemetrexed and combination segment.
Evaluation of combination therapies over monotherapy is one of the main factors creating a positive impact on the Pemetrexed and combination drug type segment.
Related Reports:
About Us:
Persistence Market Research’s Expertise in Life Sciences and Transformational Health
Our expert team of industry analysts comprising management graduates, medical professionals, engineers, and project managers provides insights on emerging therapy areas, diagnostic tools, medical devices and components, reimbursement and market access, biotechnology, and life science research products and services to equip decision-makers with sound inputs and strategic recommendations. Click here to learn more about how we zero in on the critical aspects of this industry.
Contact Us:
Persistence market research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com