The immunoassay interference blocker-based market is expected to reach US$ 455 Mn by the end of 2031, with sales revenue expected to register 6.7% CAGR.
As per Persistence Market Research’s latest revised industry analysis, the global immunoassay interference blocker market was valued at US$ 223 Mn in 2020, and is expected to exhibit a CAGR of 6.7% over the forecast period (2021-2031).
Click Here to Get Free Sample Copy of This Report@https://www.persistencemarketresearch.com/samples/24343
Substances that modify quantifiable concentration of analytes or change antibody binding can conceivably result in immunoassay interference. Prevalence of interference is usually less in assays holding blocking agents, which counteract or inhibit the interference. Detection of interference may involve usage of a different assay, or quantification before and after treatment with supplementary blocking reagents.
Growing prevalence of chronic diseases and increasing aging population across the world are factors that are expected to surge the need for biochemical testing for the management of chronic diseases. Increase in aging population is likely to escalate demand for diagnostic kits and reagents for the detection of age-related as well as other chronic conditions. This is expected to drive the immunoassay interference blocker market over the forecast period of 2021 to 2031.
Governments around the world are supporting the growth of specialty medical diagnostic reagents, which further drives research & development. Immunoassay interference blocker kits and reagents play a vital role in preventing unnecessary interference generated while preforming an immunoassay-based disease detection test. Hence, an increase in growth of immunoassay interference blockers has been witnessed due to rising government support and funding.
Key manufacturers of immunoassay interference blockers have been focusing on expansion and new product launch programs to increase their consumer base in different geographies.
- In 2019, Bio Rab Laboratories announced the launch of an innovative test to aid in the diagnosis of Lyme disease, with the FDA clearance of the bioplex 2200 Lyme total assay.
- In 2021, Thermofisher Scientific planned for its manufacturing site expansion in the region of Mebane, N.C. USA.
Key Takeaways from Market Study
- Antibody interference blockers hold the largest market share as compared to other products, expanding at a CAGR of 6.4%. A similar trend is expected to continue, primarily due to their high efficiency and reliable assay outcomes.
- The application segment is led by antibody capture assays with a share of 39.8% in 2020, owing to high, precise, and accurate results with least interference obtained via immunoassay interference blockers.
- Academic and research institutes dominated the end user segment with a share of around 39.5% in 2020. This segment is expected to grow two-fold by the end of 2031 due to increasing usage of immunoassay interference blockers in research activities to introduce novel applications.
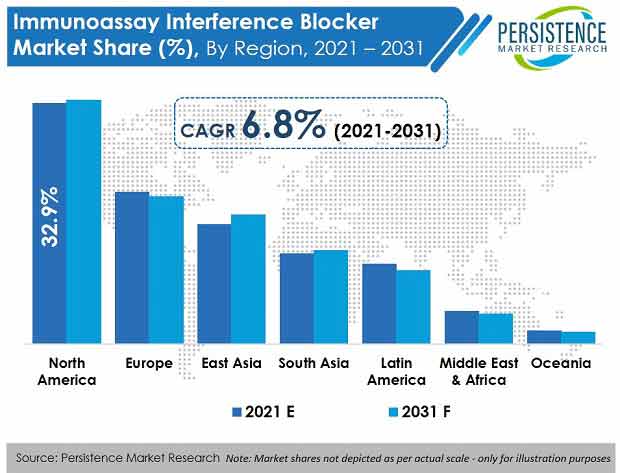
- By region, North America is slated to be the largest regional market with a value share of 33.3% at the end of the forecast period, due to constant clinical efforts to establish advanced diagnostics biologics and new drug discovery in the region.
“Increasing healthcare R&D spending and government support, continual technological advancements, and growing demand for better diagnosis are boosting demand for immunoassay interference blockers,” says an analyst of Persistence Market Research.