Orally Disintegrating Tablet Market 2022
The Orally Disintegrating Tablet Market is slated to grow at a gracious rate of 10%, reaching US$ 12 Bn by the year 2022. With a value-oriented approach being the need of the hour, the healthcare vertical is likely to go the technologically advanced way in the next 10 years. With Big Data, and AI comprising these advancements, the healthcare vertical is bound to create greater strides going forward.
Improvements in drug delivery mechanism offering quicker onset of action with superior bioavailability per dose have transformed characteristics of orally administered drugs with the development of orally disintegrating tablets (ODTs). The global orally disintegrating tablets market was valued at nearly US$ 12 Bn in 2018 and will exhibit a solid CAGR during the forecast period (2019 – 2029).
Get Free Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/14572
There has been growing preference among consumers and patients for orally disintegrating tablets attributed to increasing applications in treatment of diseases related to central nervous system (CNS), gastrointestinal (GI), cardiovascular disorders, and allergy. Bayer’s acquisition of Merck’s consumer care unit was aimed at gaining shares in ODTs. Claritin, an ODT received through this acquisition, was the largest revenue generating drug for allergy in the U.S. that year.
Company Profiles:
Teva Pharmaceutical Industries Ltd., Novartis AG, AstraZeneca, Mylan N.V., Pfizer Inc., Johnson & Johnson Services, Inc., F. Hoffmann-La Roche Ltd.,Merck & Co., Inc., Bausch Health, GlaxoSmithKline plc., Sun Pharmaceutical Industries Ltd., Bayer AG, Eli Lily and Company, Dr. Reddy’s Laboratories Ltd., Takeda Pharmaceutical Company Limited
Key Manufacturers Exploring New Business Models
The global orally disintegrating tablets market is fragmented with low entry barriers inspiring generic manufacturers to tap market opportunities in the form of ANDA, arising from the expiry of patent regime of approved drugs. For instance, Mylan N.V. launched Lansoprazole DR ODT, a generic version of Takeda’s Prevacid® SoluTab DR ODT. The entry of The Himalaya Drug Company LTD. and Banyan Botanicals with new herbal ODT product offerings in next few years is expected to increase product lines and extension in ODTs market.
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/14572
Key Takeaways – Orally Disintegrating Tablets Market Study
- CNS diseases remain the primary area of application for orally disintegrating tablets. Researchers and healthcare providers are increasingly focusing on medical conditions such as encephalitis, autism, Alzheimer’s disease, schizophrenia, depressive disorders and others to increase the application of orally disintegrating tablets.
- Formulation process of orally disintegrating tablets is usually patented by manufacturers depending on the technology employed during the production such as lyophilization, freeze drying, and floss formation. Many technologies such as ZYDIS®, LYOC® and QUICKSOLV® are dependent on freeze drying method.
- Easy disintegration of orally disintegrating tablets in saliva within a few seconds and accuracy of dose as compared to liquid forms and chewable tablet are expected to increase the adoption of ODTs in diseases such as ulcers of throat.
Attributed to consumers facing swallowing problems, bitter taste of drugs, and patient incompliance, manufacturers are focusing to improve drug formulations. This would help them gain an edge in terms of innovation, further favoring the growth of orally disintegrating tablets market.
Report Inclusions
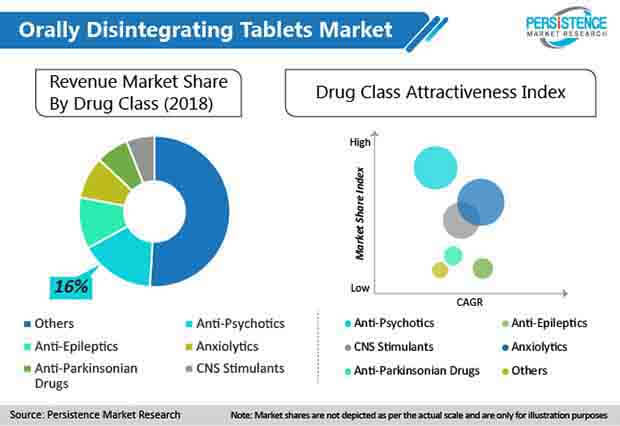
Persistence Market Research offers a unique perspective and actionable insights on the Orally Disintegrating Tablets landscape in its new study, presenting historical demand assessment from 2014 – 2018 and projections from 2019 – 2029 on the basis of drug class (anti-psychotics, anti-epileptics, CNS stimulants, anxiolytics, anti-Parkinsonian drugs, anti-hypertensives, NSAIDS, anti-allergy drugs, proton pump inhibitors), disease indication (CNS diseases, GI diseases, allergy, CVS disorders), distribution channel (hospital pharmacies, retail pharmacies, drug stores, online pharmacies), and five key regions.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/14572
Orally Disintegrating Tablet Market – Report Highlights
- A detailed overview of parent market of Orally Disintegrating Tablet Market
- Changing Orally Disintegrating Tablet Market dynamics in the industry
- In-depth segmentation of the Orally Disintegrating Tablet Market
- Historical, current, and projected Orally Disintegrating Tablet Market size regarding volume and value
- Recent industry trends and developments in Orally Disintegrating Tablet Market
- Competitive landscape of the Orally Disintegrating Tablet Market
- Strategies for key players and products offered
- Potential and niche segments, geographical regions exhibiting promising growth
- A neutral perspective on Orally Disintegrating Tablet Market performance
Must-have information for Orally Disintegrating Tablet Market players to sustain and enhance their market footprint
About us:
PersistenceMarketResearch is an esteemed company with a reputation of serving clients across domains of information technology (IT), healthcare, and chemicals. Our analysts undertake painstaking primary and secondary research to provide a seamless report with a 360 degree perspective. Data is compared against rep/uted organizations, trustworthy databases, and international surveys for producing impeccable reports backed with graphical and statistical information.
Contact us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com